Research |
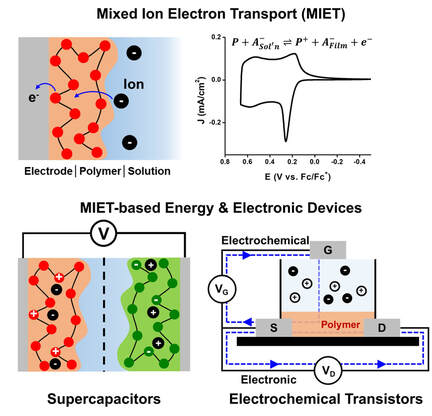
Mixed Ion Electron Transport (MIET) of Conjugated Polymers
The invention of pi-conjugated polymers by Alan Heeger, Alan MacDiarmid, and Hideki Shirakawa led to the remarkable development of solid-state organic electronics including organic solar cells, transistors, light emitting diodes, and thermo-electrics. Recently, significant attention has been paid to expand their utility in a liquid environment for potential applications such as sensors, bioelectronics, energy conversion/storage devices, and neuromorphic computing. The Mixed Ion Electron Transport (MIET) process (or electrochemical activity) of conjugated polymers is a keystone making those applications feasible. The abilities of ionic and electronic conduction in conjugated polymers largely depend on chemical and physical properties of the materials. A systematic understanding MIET process at the conjugated polymer films would pave a road to build a design strategy of the materials and develop wide range of conjugated polymer-based electrochemical systems. Our group will mainly focus on 1) unveiling structure-property relationship by combination of electroanalysis, spectroscopy, and synchrotron characterizations, 2) design and synthesis of new materials suitable for MIET process, and 3) developing next generation energy and/or electronic devices.
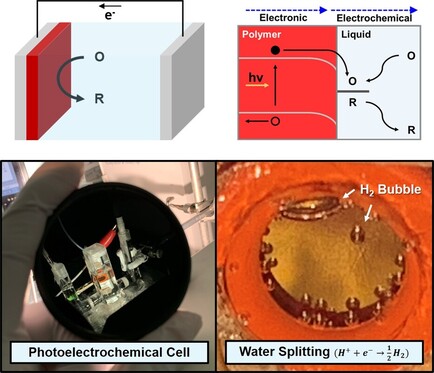
Photoelectrochemistry of Organic Semiconductors
The Choi Group will seek to develop artificial photosynthesis systems to produce fuels using organic semiconductors. Clean and renewable energy is highly required nowadays to meet the increasing global energy demand and environmental concerns, and solar energy is one of the renewable energy resources for photoelectrochemical water splitting. To date, although many inorganic semiconductors have demonstrated photoelectrochemical water splitting, systems that can produce hydrogen at a price competitive with fossil fuels remain elusive. Therefore, a new generation of high-performance and stable materials based on earth-abundant elements, low processing cost, and low toxicity is needed for the storage of solar energy and a carbon neutral chemical economy. Organic semiconductors are potential candidates satisfying the criteria that materials consist of earth abundant materials and the organic semiconductor-based devices can be prepared by low cost and large-scale processing techniques such as inkjet and roll-to-roll printings. Moreover, tunable chemical structures and electronic properties (i.e. bandgap) of organic semiconductors afford an opportunity to develop high performance organic photoelectrochemical (OPEC) systems for hydrogen production.
Organic semiconductors have been extensively studied in solid-state organic photovoltaics. The power conversion efficiency (solar-to-electricity) has surpassed 17% by optimization of molecular structures and engineering of photovoltaic devices. However, relatively fewer advances have been made on OPEC research due to the discipline barrier between organic electronics and electrochemistry. Our goal is to fill the gap and exploiting semiconducting polymers for artificial photosynthesis. We are foremost interested in (1) engineering surface and morphology of organic semiconductor films, (2) photoelectrochemical analysis using advanced electrochemical tools (i.e. scanning electrochemical microscopy and intensity modulated photoelectrochemial spectroscopy), and (3) develop low cost, scalable, and efficient organic semiconductor-based artificial photosynthesis systems.
Organic semiconductors have been extensively studied in solid-state organic photovoltaics. The power conversion efficiency (solar-to-electricity) has surpassed 17% by optimization of molecular structures and engineering of photovoltaic devices. However, relatively fewer advances have been made on OPEC research due to the discipline barrier between organic electronics and electrochemistry. Our goal is to fill the gap and exploiting semiconducting polymers for artificial photosynthesis. We are foremost interested in (1) engineering surface and morphology of organic semiconductor films, (2) photoelectrochemical analysis using advanced electrochemical tools (i.e. scanning electrochemical microscopy and intensity modulated photoelectrochemial spectroscopy), and (3) develop low cost, scalable, and efficient organic semiconductor-based artificial photosynthesis systems.
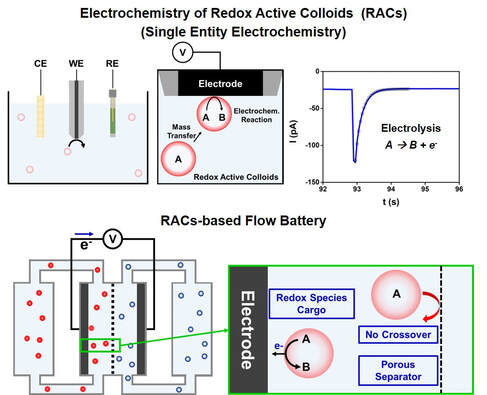
Electrochemistry of Redox Active Colloids
Redox flow batteries are rechargeable batteries that energy is stored in electrolyte solutions (so called anolyte and catholyte) that are pumped through each electrode of flow cells separated by a membrane. Their unique cell architecture brings about easy scalability and large energy storage capacity by increasing the tank volume. Research have been focused on metal salts-based redox flow batteries, and especially vanadium redox flow battery showed good reversibility and large power density. However, commercialization of metal salts-based redox flow batteries is hindered by shortcomings including toxicity and scarcity of metal salts and high cost (500-700 USD per square meter) of ion exchange membrane. Next generation materials are needed for safe and affordable redox flow battery technology.
Redox active colloids are considered as new types of materials for redox flow battery based on their low toxicity and electrochemical redox activity. One of the biggest advantages of using redox active colloids for flow battery is the large sizes of the colloids, thereby not requiring expensive ion exchange membranes (i.e. Nafion). Instead, a simple porous separator (size exclusion) can be utilized to inhibit crossover of active species, improve cycling stability, and reduce flow battery cost.
Redox active colloids are considered as new types of materials for redox flow battery based on their low toxicity and electrochemical redox activity. One of the biggest advantages of using redox active colloids for flow battery is the large sizes of the colloids, thereby not requiring expensive ion exchange membranes (i.e. Nafion). Instead, a simple porous separator (size exclusion) can be utilized to inhibit crossover of active species, improve cycling stability, and reduce flow battery cost.
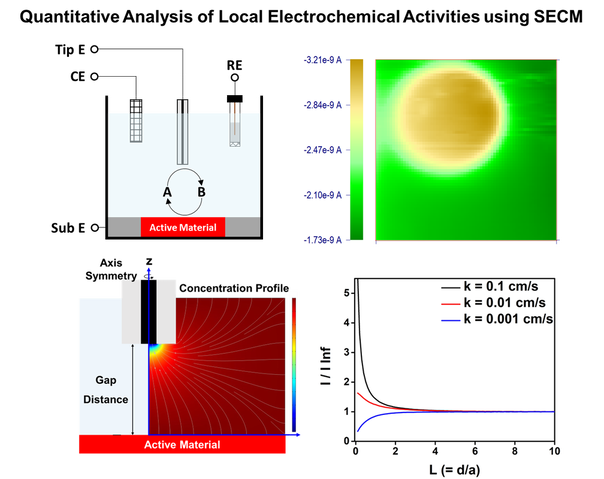
In-Situ Analysis of Electrochemical Reactions
Electrochemical processes offer sustainable and environmentally benign routes to solve energy problems. Challenges in many electrochemical systems is to design materials (i.e., electrocatalysts and electrolytes) for a specific electrochemical reaction with high selectivity and yield. Establishing rational design strategies of materials is often hindered by limited understanding of the electrochemical reactions at molecular level. We focus on investigating dynamics and kinetics of electrochemical reactions at molecular level quantitatively by using in-situ electroanalytical techniques (i.e., scanning electrochemical microscopy and spectroelectrochemistry). The in-situ investigation can be expanded to solve electrochemical problems in diverse research areas including energy storage (i.e., electrocatalysis and battery) and conversion (i.e., photocatalysis), biology (i.e., electroactive microorganisms), and corrosion.